
Your immune system protects you from threats like viruses, bacteria, and even cancer. However, some cancer cells have developed clever ways to avoid detection. They disguise themselves to look like normal cells, effectively tricking the immune system into ignoring them.
Immune checkpoint inhibitors (ICIs) are a type of immunotherapy that helps the immune system recognize and attack these hidden cancer cells.
Throughout this post, we’ll refer to several key immune system proteins and therapies using acronyms. Here’s a quick reference table to help keep things clear:
| Acrynom | Stands For | What It Means |
ICI |
Immune Checkpoint Inhibitor |
A drug that blocks immune “off switches” (checkpoints), helping T cells attack cancer. |
PD-1 |
Programmed Death-1 |
A checkpoint protein on T cells that tells them to slow down or stop—helps prevent attacking healthy cells. |
PD-L1 |
Programmed Death Ligand-1 |
A checkpoint protein on normal and cancer cells that binds to PD-1, sending a “don’t attack” signal. |
CTLA-4 |
Cytotoxic T-Lymphocyte–Associated Protein 4 |
Another immune checkpoint protein that helps regulate T cell activity and avoid overreaction. |
irAE |
Immune-related Adverse Event |
A side effect of immunotherapy where the immune system mistakenly attacks healthy tissue. |
mirSNP |
MicroRNA Single-Nucleotide Polymorphism |
An inherited genetic change that can alter how microRNAs regulate genes—can affect treatment response or side effect risk. |
HLA |
Human Leukocyte Antigen |
A molecule on the surface of cells that shows protein fragments (antigens) to T cells. This allows the immune system to recognize which cells are healthy and which are infected or abnormal. |
How Does the Immune System Work?
To understand how ICIs work, it’s helpful to explore first how the immune system normally detects and responds to threats.
The immune system is a highly coordinated network of organs, cells, and molecules that work together to eliminate harmful invaders, including viruses, bacteria, fungi, and even allergens such as pollen or pet dander. Its protective status, known as immunity, has two main types: innate immunity and adaptive immunity. Although each type of immunity has its own duties, both work in concert to protect your health.
Your Body’s First Line of Defense
Innate immunity is your first line of defense. It includes physical barriers such as skin and the lining of your gut, bodily secretions like tears and stomach acid, and certain immune cells that respond quickly to invaders.
Your Body’s Secret Weapon: The Adaptive Immune Response
When invaders get past the body’s front-line defenses, the adaptive immune system responds in two ways: humoral immunity, which uses B cells to produce antibodies against circulating invaders, and cell-mediated immunity, which relies on T cells to eliminate infected or abnormal cells. While this antibody response is essential for defending against many external threats, the immune system relies more heavily on T cells, especially killer T cells, to detect and eliminate infected or cancerous cells.
Cell-Mediated Immunity Explained: How T Cells Recognize Cancer Cells
Killer T cells constantly patrol the body, scanning cells for signs of trouble. But how do they tell a healthy cell from a cancerous or infected one?
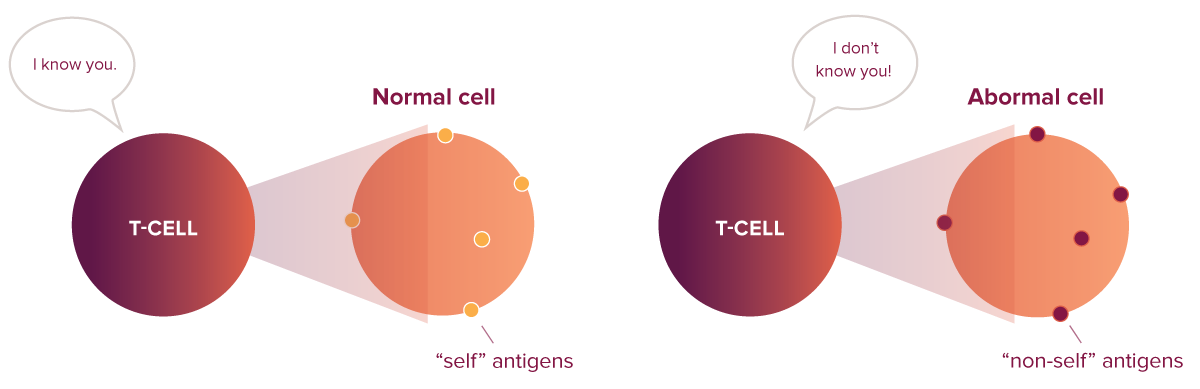
Every cell in your body reveals what’s happening inside using molecules called human leukocyte antigens (HLAs), which present antigens on the cell’s surface. Healthy cells present “self” antigens—protein fragments that a normal cell has made—to signal that everything is okay.
In contrast, infected or cancerous cells display “non-self” antigens—protein fragments that are not made from a normal cell. The HLA-antigen combination works like a barcode: Killer T cells “scan” it to decide whether a cell is healthy or sick. When killer T cells scan a “non-self” barcode, they recognize the threat and mount an aggressive attack to destroy the abnormal or infected cell.
Immune Homeostasis: The Goldilocks Principle
So, how does the immune system know when to respond and when to stop?
Immune homeostasis refers to the balanced state of the immune system, where immune responses are neither too strong nor too weak—they should be “just right.” If the immune system is underactive, infections or cancer can spread. If it’s overactive, it can damage the body and lead to autoimmune diseases.
Read our blog post to learn more about autoimmune diseases.
Communication between immune cells is one way the immune system maintains this balance. Immune cells send and receive special signals or make direct contact with other cells to share information. These signals help the cells work together, ensuring that responses are properly regulated.
Checkpoint Molecules: The Immune System’s Safety Switch
The immune system has built-in “safety switches” called checkpoint proteins. These proteins help keep T cells from attacking healthy cells by mistake.
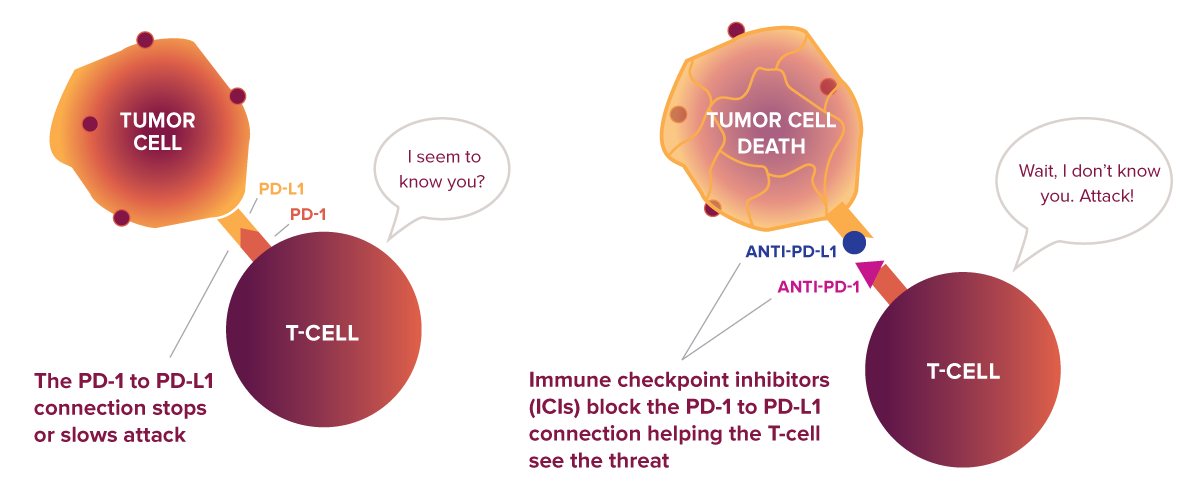
One checkpoint protein is called programmed death-1 (PD-1), which is on the surface of killer T cells. If a cell the T cell is evaluating has a matching protein called programmed death ligand-1 (PD-L1), the two connect—like a key fitting into a lock. This connection sends a signal to the T cell to slow down or stop, preventing it from harming healthy cells and maintaining immune homeostasis.
But cancer cells can hijack this system.
How Cancer Evades the Immune System
Your immune system likely detects and destroys cancerous cells many times throughout your life—before they ever become a problem. Killer T cells can recognize tumor-specific antigens (“non-self” antigens) on cancer cells and eliminate them just as they do virus-infected cells.
However, some cancers are now known to take advantage of the immune system’s “safety switches.” They override the usual recognition process by producing an excess of PD-L1, the protein that healthy cells use to send a “don’t attack” signal to T cells. When a killer T cell encounters one of these cancer cells, because of the overexpressed PD-L1, the T cell doesn’t attack, despite the cancer cell being abnormal.1
This evasion strategy is used by cancers such as melanoma,2 non-small cell lung cancer,3 head and neck cancer,4 breast cancer,5 pancreatic cancer,6 ovarian cancer,7 and likely many more.
What are Immune Checkpoint Inhibitors?
Immune checkpoint inhibitors (ICIs) are a type of immunotherapy that helps the immune system do its job. These drugs block either PD-1 (on killer T cells) and/or PD-L1 (on cancer cells). When one of these checkpoint proteins is blocked, the killer T cell no longer receives the “stop” signal. Instead, it stays active and can attack the cancer.
Checkpoint Inhibitors: A New Era in Cancer Treatment
ICIs have launched a new era in cancer treatment. The FDA approved the first ICI, ipilimumab (which blocks CTLA-4, another checkpoint protein), for melanoma in 2011. Since then, newer ICIs such as nivolumab and pembrolizumab that block PD-1 are now used to treat a wide range of cancers, including lung, breast, and head and neck cancers.
These treatments have led to major breakthroughs, especially in cancers that used to be very hard to treat. For example, in a clinical trial for metastatic melanoma, patients who received ipilimumab had a one- and two-year survival rate of 45.6% and 23.5%,8 whereas the median survival rate was less than a year with traditional treatments.9 This was the first time that long-term survival became a real possibility for many patients with this aggressive form of skin cancer.
Researchers are exploring new ways to expand the benefits of ICIs:
- Combination therapies that pair ICIs with chemotherapy or radiation therapy for improved outcomes, or combinations of different ICIs.
- New checkpoint targets like TIM-3 and TIGIT to help patients who don’t respond to current therapies.
Still, not everyone benefits—and side effects can be serious.
Predicting Who May Benefit
Only about 20–40% of patients respond to ICIs, while nearly half either show no response or develop resistance.10-12 How can we identify patients who are more likely to respond to treatment?
One approach involves using tumor-based biomarkers, such as measuring the expression level of PD-L1 on cancer cells. Higher levels of PD-L1 are generally linked to better responses to ICIs. For instance, studies on patients with non-small cell lung cancer showed that those with high PD-L1 levels exhibit a significantly better response to anti-PD-1 therapy and experience longer progression-free survival compared to those with moderate levels.13,14
Predicting Who May Experience Side Effects
Not surprisingly, because ICIs remove the “brakes” on the immune system, they can also overstimulate it, causing it to attack healthy tissue. These side effects, known as immune-related adverse events (irAEs), affect approximately 30% of patients and may involve the lungs, thyroid, gut, and other tissues.15 Most are treatable, but some can be severe.
Unlike some treatment responses, side effects appear to be more tied to a person’s inherited genetics than tumor characteristics, because these side effects are in their normal tissues, not their cancer tissues. For example, how someone’s body responds to stress—like DNA damage or an immune response from therapies—is unique to their genetic makeup. Dr. Joanne Weidhaas and her colleagues at UCLA are researching inherited genetic variants in microRNAs, known as mirSNPs, to predict the risk of irAEs associated with ICIs.
Their earlier research identified mirSNPs that could predict which patients are more likely to develop irAEs from anti-PD-1 or anti-PD-L1 therapies, regardless of cancer type.16,17 Currently, they’ve identified other mirSNPs that not only can predict irAEs but also the response to anti-CTLA-4 therapy in melanoma patients.18 Together, this work suggests that mirSNPs may help identify patients who are both more likely to respond to ICIs and more likely to develop side effects—information that could guide treatment decisions before therapy begins.
Empowering the Immune System to Outsmart Cancer
ICIs have revolutionized treatment for some hard-to-treat cancers by strengthening the body’s natural defenses. But there’s still more work to do. Scientists are learning to predict who will benefit from these treatments, how to avoid irAEs, and how to combine them with other therapies. The goal is to make these treatments more effective and safer for a broader range of patients, moving us closer to a future where more cancers can be treated with the help of the immune system.
References
- https://doi.org/10.1084/jem.20160801
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3549297/
- https://pubmed.ncbi.nlm.nih.gov/27310809/
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.01721/full
- https://www.nature.com/articles/s41598-019-50898-3
- https://www.nature.com/articles/s41392-020-0144-8
- https://www.sciencedirect.com/science/article/pii/S0090825824000477
- https://www.nejm.org/doi/full/10.1056/NEJMoa1003466
- https://pubmed.ncbi.nlm.nih.gov/19445576/
- https://ascopubs.org/doi/full/10.1200/JCO.19.00934
- https://link.springer.com/article/10.1186/s40425-018-0382-2
- https://www.sciencedirect.com/science/article/pii/S092375341931110X
- https://pubmed.ncbi.nlm.nih.gov/31435660/
- https://link.springer.com/article/10.1007/s12325-019-01057-7
- https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2021.683419/full
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8804679/
- https://ascopubs.org/doi/10.1200/JCO.2025.43.16_suppl.2661
- Weidhaas JB, et al. Germline microRNA-based signatures predict toxicity and response to anti-CTLA4 therapy. In Press.
Top Questions Patients Ask About Checkpoint Inhibitors
1) How do checkpoint inhibitors cause the immune system to attack healthy cells?
Checkpoint inhibitors work by removing the “brakes” on the immune system so it can attack cancer cells. But when these brakes are released, the immune system can sometimes go too far and target healthy tissues—like the lungs, thyroid, or gut—causing what doctors call immune-related adverse events (irAEs).
2) Can you predict who will get severe side effects from immunotherapy before treatment?
Researchers are working on it. While not yet routine in the clinic, new studies show that inherited genetic markers—like microRNA variants (mirSNPs)—may help predict which patients are more likely to develop severe side effects from checkpoint inhibitors before they even start treatment.
3) What can be done to manage or prevent irAEs during treatment?
Doctors closely monitor patients for early signs of side effects, such as changes in breathing, digestion, or energy levels. Many irAEs can be managed by stopping treatment and treating with steroids or other medications if caught early. Open communication between patients and their care team is key to preventing more serious complications.
4) Will new biomarkers help make checkpoint inhibitor treatments safer for everyone?
That’s the goal. By using genetic biomarkers and other predictive tests, doctors hope to identify patients at higher risk of side effects before they start treatment. This could help tailor therapy plans, reduce complications, and make immunotherapy safer and more effective for more people.




Leave a Reply