Access PROSTOX Testing
Understanding your PROSTOX results
Once your results are ready, you will need to discuss them with a physician.
PROSTOX ultra
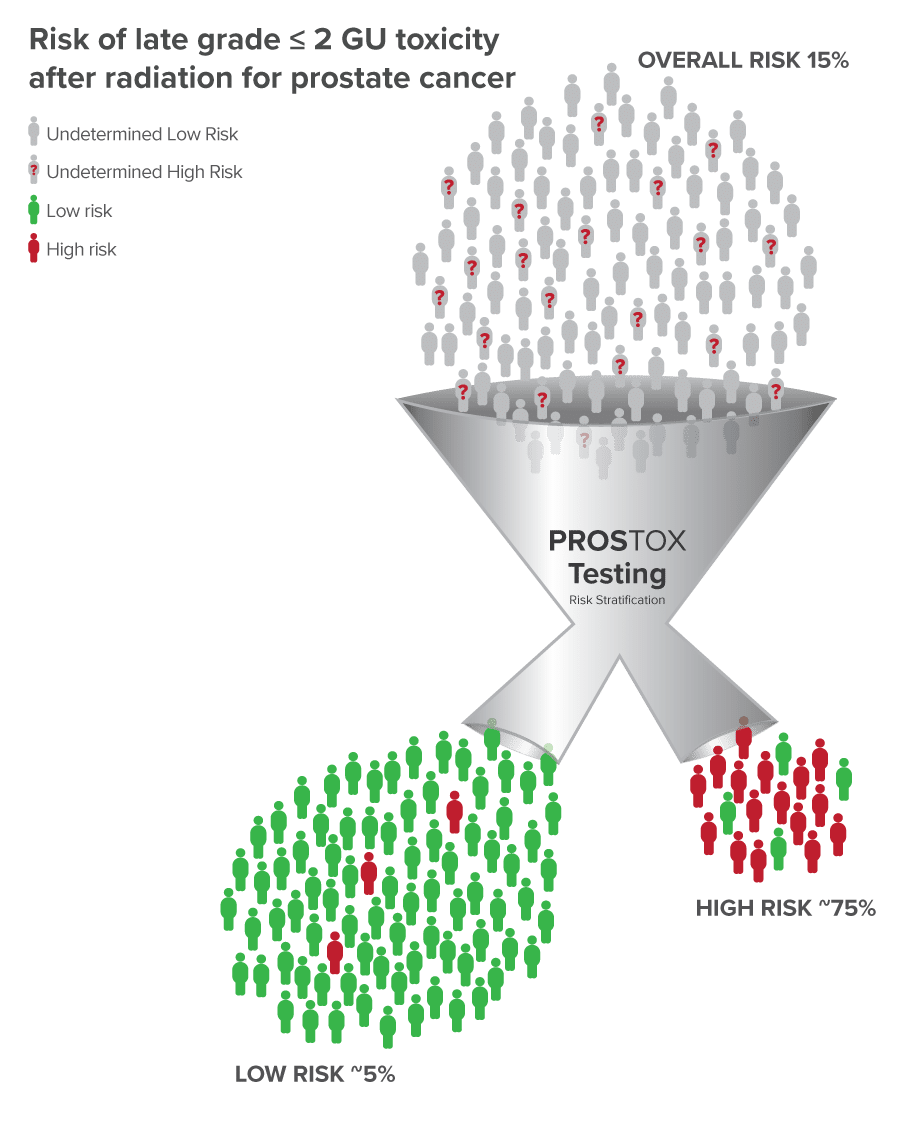
- If a patient is found to be High Risk, they have a ~75% chance of developing late GU toxicity from SBRT to the prostate, whereas if they are Low Risk they have less than a 5% risk.1,2
PROSTOX CFRT
- If a patient is found to be High Risk, they have a ~72% chance of developing late GU toxicity from CFRT to the prostate, whereas if they are Low Risk they have less than a 7.5% risk.3
Questions? Read our PROSTOX FAQ or Contact us
Frequently asked questions about MiraDx PROSTOX testing through MiraKind
General questions about PROSTOX
Accessing PROSTOX through MiraKind
Taking the test
Participating in our research registry
Disclosure: Any discussion of medical management options is for general informational purposes only and does not constitute a medical recommendation. All medical management decisions should be made based on consultation between each patient and his or her healthcare professional.
References
- Kishan AU, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Network Open 2019;2:e188006.
- Weidhaas, JB, et al. MicroRNA-based biomarkers of the radiation response in prostate cancer. Journal of Clinical Oncology 38, no. 6_suppl (February 20, 2020) 163-163. DOI: 10.1200/JCO.2020.38.6_suppl.163.
- Kishan AU, et al. Germline variants disrupting microRNAs predict long-term genitourinary toxicity after prostate cancer radiation. Journal for Radiotherapy and Oncology 2022;167:226-232. doi: 10.1016/j.radonc.2021.12.040.
- Kishan AU, Marco N, Ma TM, Steinberg ML, Sachdeva A, Cao M, Ballas LK, Rietdorf E, Telesca D, Weidhaas JB. Application of a genetic signature of late GU toxicity in SCIMITAR, a Post-op SBRT trial. Clin Transl Radiat Oncol. 2023 Feb 8;39:100594. doi: 10.1016/j.ctro.2023.100594. PMID: 36880064; PMCID: PMC9984404.


