How can inherited genetic differences help predict who’s more likely to benefit from cancer treatment—or experience severe side effects?
That question is at the heart of two new studies we recently shared on sarcoma and immunotherapy. These early findings were presented at ASCO 2025—the annual meeting of the American Society of Clinical Oncology and one of the world’s largest cancer research events. Each year, more than 40,000 experts gather at ASCO to exchange new insights and discoveries, and we were excited to be a part of it.
Dr. Joanne Weidhaas was there to present collaborative research with UCLA on a class of inherited genetic markers called microRNA single-nucleotide polymorphisms (mirSNPs), which can help predict patient response and side effects to cancer therapies such as radiation and immunotherapy.
Why is this research important? Predicting the outcome of cancer treatment is always a challenge—whether a given patient will experience serious side effects later on or if the cancer comes back. By understanding the risks before treatment begins, doctors and patients can work together to choose the safest, most effective care plan.
A Crash Course on mirSNPs
Your body has thousands of microRNAs (miRNAs)—small molecules that help regulate how the body responds to stress, such as DNA damage, caused by illness, injury, or even cancer treatments.
Many people are born with mirSNPs—variations in miRNA binding sites or within the miRNAs themselves—that can change how these molecules work. Some have little effect, while others can raise cancer risk or alter treatment response.
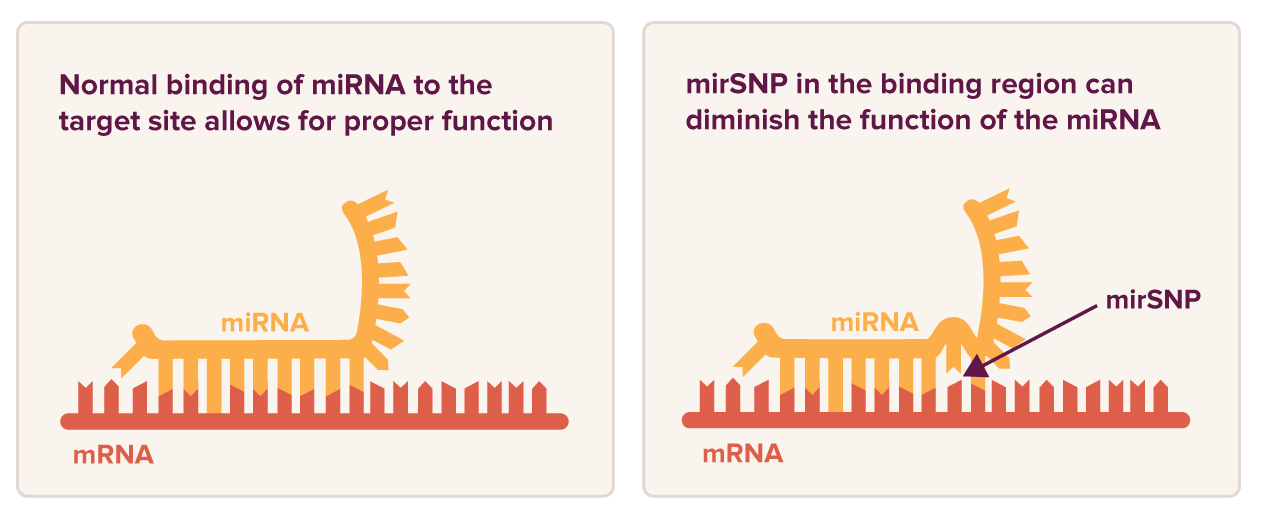
Want to know about your genetics and how miRNA variants can impact your health? Check out our Genetics 102 page.
Dr. Weidhaas co-discovered the well-known mirSNP, the KRAS-variant, in 2006. It’s linked to a higher risk of certain cancers,2-7 including breast cancer, and can affect how patients respond to treatment.4,8-20
Since then, Dr. Weidhaas has studied mirSNPs like the KRAS-variant and those making up the test PROSTOXTM Ultra, to understand how these genetic differences impact the risk of cancer and treatment-related side effects.
The two studies presented at ASCO investigate how other mirSNP panels can predict response and toxicity-related outcomes in two very different treatment settings—radiation for sarcoma patients and immunotherapy for all cancer types.
Study #1: Making Radiation Therapy Safer for Sarcoma Patients

Surgery is the main treatment for soft tissue sarcoma (STS), a type of cancer that develops in the body’s connective tissues. Radiation therapy is often used before or after surgery to shrink the tumor to allow limb-sparing surgery (keeping the leg or arm that has the sarcoma). However, not everyone tolerates radiation well. It can cause significant side effects, such as problems with wound healing after surgery. Additionally, the standard radiation schedule—spread out over several weeks—can be difficult for some patients to complete.
In a previous collaborative study with UCLA, a shorter, high-dose type of radiation known as stereotactic body radiation therapy (SBRT) was found to be both practical and effective for treating STS. However, concerns about potential side effects have made doctors cautious about using it widely.
In response to these concerns, a follow-up study of over 100 patients with STS were treated with SBRT instead of the traditional, longer approach. By examining the genetic profiles of these patients, specific mirSNP panels were identified and linked to four different outcomes:
- Late radiation toxicity—any life-altering toxicity that occurred after treatment
- Major wound toxicity—difficulty with the site of surgery healing
- Distant failure—cancer spreading elsewhere after treatment
- Pathological response—effectiveness of radiation in destroying cancer cells
By analyzing these mirSNPs, researchers can begin to understand how a person’s genes may influence their response to radiation therapy and their risk of side effects and even their risk of cancer spreading.
Although this research is still in progress, the findings offer a promising step toward more personalized treatment strategies for STS. By identifying which patients are most likely to benefit from SBRT and which may face greater risks, doctors and patients can make more informed decisions—ultimately improving outcomes and reducing unnecessary harm.
Read more about the study here.
Study #2: Predicting Immune-Related Side Effects from Immunotherapy
Immune checkpoint inhibitors (ICIs), like anti-PD1 drugs, have changed the way we treat cancer by helping the immune system recognize and attack tumor cells. They work by blocking signals that normally keep immune responses in check—essentially taking the brakes off the immune system. This heightened immune activity can sometimes go too far, leading to immune-related adverse events (irAEs) in about 30% of patients.21 These side effects happen when the immune system starts attacking healthy organs instead of just the cancer. While some irAEs can be managed, others are severe and cause permanent damage that cannot be reversed.
Earlier research with UCLA identified mirSNPs that could predict which patients are more likely to develop side effects from anti-PD1 or anti-PDL1 therapies, regardless of cancer type. Building on that research, this study developed new genetic models that predict::
- Early toxicity: Predicts irAEs that may appear within the first five treatments
- Late toxicity: Predicts irAEs that tend to develop after about 15 treatments
These models could help identify not just who is at risk of irAEs but also when they might occur. This kind of insight could provide early detection, faster management, and the potential to avoid irAEs altogether.
Read more about the study here.
A Step Toward Safer, Smarter Cancer Treatment
Both studies showcase the growing role of mirSNP-based genetic tools in personalizing cancer treatment. Whether it’s identifying who might respond better to radiation or flagging patients at risk of serious side effects from immunotherapy, these studies mark a step toward better and safer, patient-specific cancer care—where cure without harm is the underlying goal.
Read this post for some of the other studies at ASCO that stood out to us at MiraKind.
References
- Papagregoriou, G. (2015). MicroRNAs in Disease. In: Felekkis, K., Voskarides, K. (eds) Genomic Elements in Health, Disease and Evolution. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3070-8_2
- Chin LJ, et al. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res. 2008;68(20):8535-8540. doi:10.1158/0008-5472.CAN-08-2129.
- Ratner E, et al. A KRAS-variant in ovarian cancer acts as a genetic marker of cancer risk. Cancer Res. 2010;70(16):6509-6515. doi:10.1158/0008-5472.CAN-10-0689.
- Paranjape T, et al. A 3′-untranslated region KRAS variant and triple-negative breast cancer: a case-control and genetic analysis [published correction appears in Lancet Oncol. 2011 Jun;12(6):522]. Lancet Oncol. 2011;12(4):377-386. doi:10.1016/S1470-2045(11)70044-4.
- Pilarski R, et al. The KRAS-variant is associated with risk of developing double primary breast and ovarian cancer. PLoS One. 2012;7(5):e37891. doi:10.1371/journal.pone.0037891.
- Kazmi HR, et al. A let-7 microRNA binding site polymorphism in the KRAS 3’UTR is associated with increased risk and reduced survival for gallbladder cancer in North Indian population. J Cancer Res Clin Oncol. 2016;142(12):2577-2583. doi:10.1007/s00432-016-2254-9.
- Gutiérrez-Malacatt H, et al. The rs61764370 Functional Variant in the KRAS Oncogene is Associated with Chronic Myeloid Leukemia Risk in Women. Asian Pac J Cancer Prev. 2016;17(4):2265-2270. doi:10.7314/apjcp.2016.17.4.2265.
- Ratner ES, et al. A KRAS variant is a biomarker of poor outcome, platinum chemotherapy resistance and a potential target for therapy in ovarian cancer. Oncogene. 2012;31(42):4559-4566. doi:10.1038/onc.2011.539.
- Graziano F, et al. Genetic modulation of the Let-7 microRNA binding to KRAS 3′-untranslated region and survival of metastatic colorectal cancer patients treated with salvage cetuximab-irinotecan. Pharmacogenomics J. 2010;10(5):458-464. doi:10.1038/tpj.2010.9.
- Zhang W, et al. A let-7 microRNA-binding site polymorphism in 3′-untranslated region of KRAS gene predicts response in wild-type KRAS patients with metastatic colorectal cancer treated with cetuximab monotherapy. Ann Oncol. 2011;22(1):104-109. doi:10.1093/annonc/mdq315.
- Ruzzo A, et al. Role of KRAS let-7 LCS6 SNP in metastatic colorectal cancer patients. Ann Oncol. 2011 Jan;22(1):234-5. doi: 10.1093/annonc/mdq472. Epub 2010 Oct 6.
- Zhang W, et al. (2011). KRAS let-7 LCS6 SNP predicts cetuximab efficacy in KRASwt metastatic colorectal cancer patients: Does treatment combination partner matter? Ann Oncol. 2011 Feb;22(2):484-5. doi: 10.1093/annonc/mdq704. Epub 2011 Jan 28.
- Smits KM, et al. (2011). A let-7 microRNA SNP in the KRAS 3’UTR is prognostic in early-stage colorectal cancer. Clin Cancer Res. 2011 Dec 15;17(24):7723-31. doi: 10.1158/1078-0432.CCR-11-0990. Epub 2011 Oct 12.
- Ruzzo A, et al. (2012). High let-7a microRNA levels in KRAS-mutated colorectal carcinomas may rescue anti-EGFR therapy effects in patients with chemotherapy-refractory metastatic disease. Oncologist. 2012;17(6):823-9. doi: 10.1634/theoncologist.2012-0081. Epub 2012 May 14.
- Ryan BM, et al. (2012). KRAS-LCS6 genotype as a prognostic marker in early-stage CRC–letter. Clin Cancer Res. 2012 Jun 15;18(12):3487-8; author reply 3489. doi: 10.1158/1078-0432.CCR-12-0250. Epub 2012 Jun 5.
- Kjersem JB, et al. (2012). Let-7 miRNA-binding site polymorphism in the KRAS 3’UTR; colorectal cancer screening population prevalence and influence on clinical outcome in patients with metastatic colorectal cancer treated with 5-fluorouracil and oxaliplatin +/- cetuximab. BMC Cancer. 2012 Nov 20;12:534. doi: 10.1186/1471-2407-12-534.
- Sebio A, et al. (2013). The LCS6 polymorphism in the binding site of let-7 microRNA to the KRAS 3′-untranslated region: its role in the efficacy of anti-EGFR-based therapy in metastatic colorectal cancer patients. Pharmacogenet Genomics. 2013 Mar;23(3):142-7. doi: 10.1097/FPC.0b013e32835d9b0b.
- Sha D, et al. (2014). Association study of the let-7 miRNA-complementary site variant in the 3′ untranslated region of the KRAS gene in stage III colon cancer (NCCTG N0147 Clinical Trial). Clin Cancer Res. 2014 Jun 15;20(12):3319-27. doi: 10.1158/1078-0432.CCR-14-0069. Epub 2014 Apr 11.
- Langevin SM, Christensen BC. (2014). Let-7 microRNA-binding-site polymorphism in the 3’UTR of KRAS and colorectal cancer outcome: a systematic review and meta-analysis. Cancer Med. 2014 Oct;3(5):1385-95. doi: 10.1002/cam4.279. Epub 2014 Jun 2. Review.
- Saridaki Z, et al. (2014). A let-7 microRNA-binding site polymorphism in KRAS predicts improved outcome in patients with metastatic colorectal cancer treated with salvage cetuximab/panitumumab monotherapy. Clin Cancer Res. 2014 Sep 1;20(17):4499-510. doi: 10.1158/1078-0432.CCR-14-0348.
- Postow M, Wolchok J. Toxicities associated with checkpoint inhibitor immunotherapy. Post TW. Updated, 2017.




Leave a Reply